Research on combination therapies involving human papillomavirus-targeted vaccines and immunotherapies continues to expand in phase 2 and 3 trials in patients with head and neck squamous cell carcinoma.

Robert L. Ferris, MD, PhD
Research on combination therapies involving human papillomavirus (HPV)-targeted vaccines and immunotherapies continues to expand in phase 2 and 3 trials in patients with head and neck squamous cell carcinoma (HNSCC). HPV-associated tumors, particularly HPV-16–positive cancers, represent a biologically distinct subgroup of HNSCC.1 These tumors exhibit distinct immune characteristics and a different tumor microenvironment compared with HPV-negative tumors. Patients with HPV-positive HNSCC have a better prognosis and dramatically improved 5-year survival (~70%) compared with their HPV-negative counterparts (~40%-50%).1,2 Patients with HPV-positive cancers present 13 years earlier at diagnosis and live longer lives. However, treatment for both HPV-related and -unrelated cancer subtypes has largely remained the same. In a step toward precision medicine, investigators are now examining ways to exploit the specific immune properties of HPV-positive tumors to target the viral origin of the disease.
The mainstay of first-line treatment for advanced HNSCC consists of surgery followed by radiotherapy with or without platinum-based chemotherapy in combination with cetuximab (Erbitux).3 Given the high survival rate in HPV-positive HNSCC, efforts have been made to deintensify treatment to reduce toxicities and the resulting comorbidities and their negative impact on patients’ quality of life.2,3 Pembrolizumab (Keytruda) and nivolumab (Opdivo) were recently approved for recurrent and/or metastatic oropharyngeal cancers (OPCs) in patients progressing on or after platinum-based chemotherapy.2,3 Pembrolizumab, in combination with platinum-based chemotherapy and 5-fluorouracil, is approved for the first-line treatment of all patients with recurrent and/or metastatic HNSCC and as monotherapy in patients whose tumors express PD-L1 with a combined positive score of 1 or more.2,3
Not withstanding the good prognosis seen with HPV-associated HNSCCs, approximately one-third of patients with this cancer subtype will develop metastatic disease and die. No targeted therapies have been developed or approved for those in the HPV-positive HNSCC subset.4 “Despite its different biology, disease characteristics, and behavior, and its viral underlying cause, HPV-associated cancer continues to be treated with the same aggressive chemoradiotherapy regimens traditionally used for HPV-negative cancers,” said Robert L. Ferris, MD, PhD, director of UPMC Hillman Cancer Center at the University of Pittsburgh in Pennsylvania, during an interview with Targeted Therapies in Oncology. “With improved survival comes increased toxicities [and] permanent treatment-related morbidities in a younger population [of patients who have] a longer lifespan [and have] overwhelmed survivorship clinics. These factors have important considerations for society and the health systems that [treat] these patients.” An important unmet medical need exists for safe and effective treatments in this population.

Nabil F. Saba, MD
Single-agent PD-1 immunotherapy, however, has demonstrated durable clinical responses and survival in a small number of patients (~20%) with advanced HNSCC.4 Investigational HPV-16–targeted vaccination induced a strong immune response, but was not effective alone at treating advanced cancers.4 Emerging strategies include combinations of PD-1/PD-L1 inhibitors with therapeutic HPV vaccines targeting non–self-antigens to activate the immune system against the tumor.5 This article explores the clinical benefits of therapeutic HPV vaccines in HPV-associated HNSCCs and the ongoing clinical trials combining HPV-targeted vaccines with immunotherapies.
Tumor Microenvironment in Head and Neck Cancers
The tumor microenvironment in advanced HNSCC is highly immunosuppressive and characterized by T-cell dysfunction, low levels of CD4- and CD8-positive T cells, increased regulatory T cells (Tregs), and disrupted antigen presentation.5 T-effector (Teff) and natural killer (NK) cells are responsible for tumor attack, whereas Tregs mediate tumor cell growth and immune suppression. HPV-positive tumors have greater levels of tumor-infiltrating lymphocytes (TILs), such as T, B, and NK cells, compared with HPV-negative tumors, and patients whose HPV-positive tumors have higher TIL levels have better out-comes than those with lower TIL levels. Within this microenvironment, outcomes also depend on the balance between levels of tumor-directed Teff cells and immunosuppressive Tregs in the TIL population.2
Therapeutic HPV Vaccination in Combination with PD-1/-L1 Immunotherapy
HPV-positive OPCs, particularly advanced cancers, develop adaptive immune evasion mechanisms through the upregulation of PD-1/PD-L1 check-points. This attenuates CD4-positive T-cell cytotoxic activity, decreases myeloid-derived suppressor cell numbers, and acts through other mechanisms. A strong rationale exists for the use of PD-1/PD-L1 inhibitors to improve efficacy and reduce toxicities during deintensification approaches.2,4,6,7
The FDA has approved prophylactic HPV vaccines for the prevention of gynecological and oropharyngeal cancers caused by infections with high-risk HPV types, including HPV-16.8,9 These vaccines stimulate the production of neutralizing antibodies and prevent HPV-associated cancers but do not target viral antigens expressed during the tumorigenic process in HPV-positive cancers.
Viral proteins are considered foreign to the immune system, or non–self-antigens, and represent ideal targets for therapeutic vaccination. HPV viral proteins E6 and E7 are the main oncogenes expressed in HPV-positive cancers that drive oncogenesis and maintain HPV-associated disease.2,5,6,10 Vaccines targeting the E6 or E7 antigens have shown efficacy in HPV-induced cervical dysplasia and investigators are now testing them in head and neck cancers.11,12 They are also evaluating the E5 HPV viral oncogene in preclinical studies, which is thought to cooperate with E6 or E7 in tumorigenesis.10,11
HPV-specific immunotherapy through targeted therapeutic vaccines triggers strong immune responses and tumor infiltration by HPV-specific T cells2; however, HPV-16–targeted vaccination alone has been ineffective in treating recurrent advanced HPV-positive cancers. An emerging area of interest is the use of HPV antigen–targeted vaccination to stimulate a proinflammatory immune response in combination with PD-1/PD-L1 immune checkpoint inhibitors to enhance the effect of these agents. The combination may increase the number of patients who respond to therapy and thus may improve outcomes in HPV-associated HNSCC.5,11
Unique Combinations: Vaccines and PD-1/PD-L1 Inhibitors
TG4001
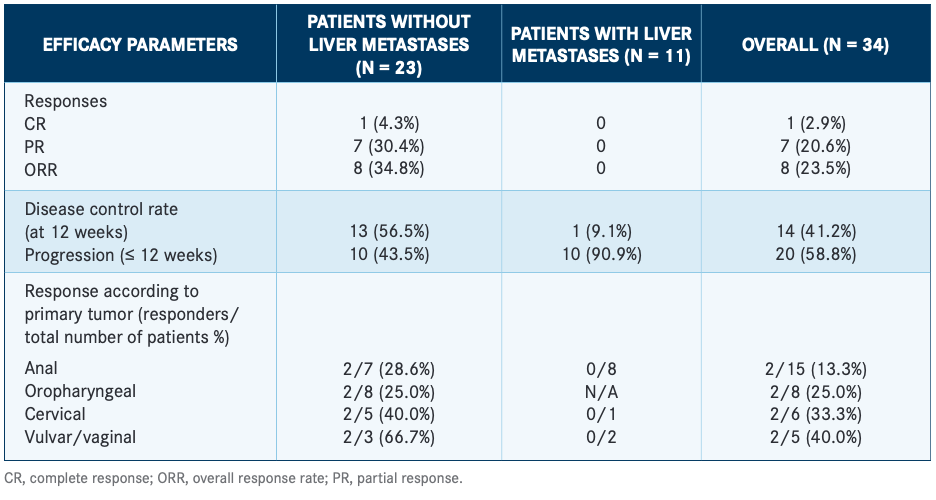
Positive results of a phase 1b/2 trial (NCT03260023) of the investigational therapeutic vaccine TG4001 (tipapkinogene sovacivec) plus avelumab (Bavencio) were recently presented at the European Society for Medical Oncology (ESMO) Virtual Congress 2020. Patients (n=34) with previously treated recurrent and/or metastatic HPV-16–positive cancers received TG4001, an HPV-16E6/E7–targeted vaccine, in combination with avelumab.13,14
The overall response rate (ORR) was 23.5% in all evaluable patients, including 7 partial responses and 1 complete response; the latter was durable. Trial investigators observed markedly different ORRs in patients with liver metastases (0%; n=11) and in those without (34.8%; n=23; TABLE).13 Treatment induced an HPV-16 E6/E7–specific T-cell response and favorable changes in the tumor microenvironment, including increases in TILs and the expression of genes related to immune system activation. The authors concluded that a larger, randomized clinical trial was warranted to confirm these findings.13,14
ISA101
In a phase 2 study, investigators examined whether the HPV-16 vaccine ISA101 could augment the efficacy of the PD-1 inhibitor nivolumab in advanced HPV-positive tumors. ISA101 triggers CD4/CD8-positiveT-cell responses and has shown efficacy in advanced gynecological cancer.7,15 Enrolled patients (n=24) had incurable HPV-16–positive gynecological and oropharyngeal tumors, of which 22 were OPCs.
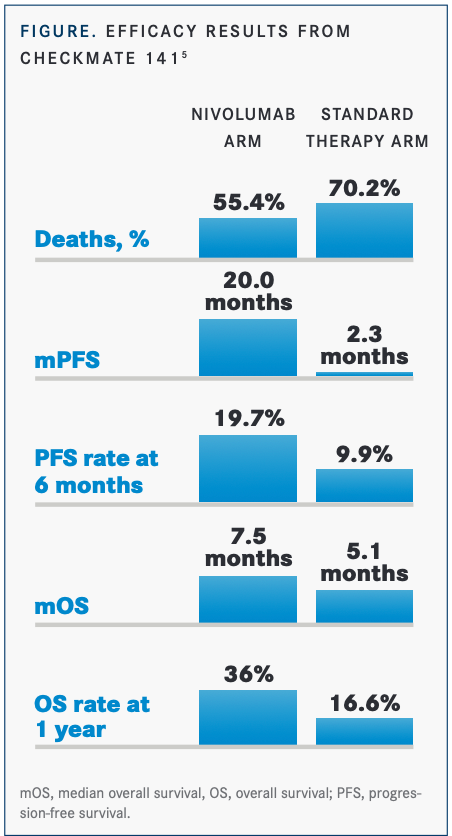
Eight of the 22 patients with OPC (36% ORR) met the primary end point of overall survival (OS) compared with the 16% ORR seen with nivolumab alone in HPV-positive patients in CheckMate-141 (NCT02105636).5 Five of 8 patients (63%) had durable responses, and the OS rate was 70% (FIGURE5). Patients toleratedthe combination of ISA101 and nivolumab well. The median OS of 17.5 months with the combination was approximately double that seen with pembrolizumab or nivolumabin subsets of patients who were HPV-16 positive.4,7,16 The study results supported the rationale that using PD-1 inhibitors to block tumor-induced immunosuppression would potentiate the cytotoxic effects of the vaccine and that HPV-16 vaccination would augment the efficacy of PD-1 inhibitors in incurable HPV-16–positive cancer. Further studies with larger numbers of patients are needed to validate this combination.17
MEDI0457
The safety and efficacy of a novel HPV-16/HPV-18–targeted DNA immunotherapy was tested in a phase 1b/2 open-label study (NCT03162224). MEDI0457 was evaluated in 22 patients with locally advanced HPV-16/HPV-18–positive HNSCC.5,11,18,19
In an earlier phase 1 pilot study (NCT00685412), women with HPV-16/HPV-18–positive high-grade cervical intraepithelial neoplasia received VGX-3100 alone, which induced E6/E7-specific immune responses and a 50% rate of tumor regression.20 With the addition of recombinant cytokine IL-12 to this HPV DNA vaccine, MEDI0457 is anticipated to boost immune responses by increasing Teff cells and overcoming HPV-driven immune evasion.5,12 VGX-3100 is now in phase 3 trials for the treatment of precancerous cervical, vulvar, and anal lesions.
In the phase 1b/2 trial mentioned above, MEDI0457induced HPV antigen-specific CD8-positive T-cell activity, generated immune infiltration of tumor tissue, and shifted the composition of TILs in the tumor microenvironment toward a proinflammatory state.5 In this trial, MEDI0457 induced persistent peripheral and tumor responses lasting up to 23 months in patients with HPV-16–positive locally advanced HNSCC, including those who had recently received concurrent chemoradiotherapy. The results of this study demonstrate the value of therapeutic HPV vaccines as a complementary strategy to PD-1/PD-L1 inhibition to improve therapeutic outcomes in HPV-associated HNSCC.5
Investigators are now evaluating MEDI0457 in combination with durvalumab(Imfinzi) in a phase 1b/2a study (NCT03162224) in 35 patients with HPV-associated recurrent or metastatic HNSCC.
JAVELIN Head and Neck 100
Interim phase 3 results of JAVELIN HEAD AND NECK 100 (NCT02952586), presented at ESMO 2020, showed that first-line treatment with avelumab plus chemoradiotherapy failed to improve progression-free survival (PFS) in patients with locally advanced HNSCC.21 The trial evaluated avelumab in patients (n=697) with histologically confirmed HNSCC who were eligible to receive chemoradiotherapy with curative intent. The PD-L1 inhibitor avelumab was added to standard high-dose chemotherapy to evaluate the impact on PFS. The trial was terminated prematurely because the futility boundary had been crossed.
Patients were randomized 1:1 (n=697) to receive avelumab 10 mg/kg every 2 weeks plus cisplatin 100 mg/m2 every 3 weeks and 7 weeks of radiotherapy at 70 Gy, followed by avelumab maintenance. In the control arm, avelumab was replaced with placebo.21 Median PFS and OS were not reached in either arm. The hazard ratio for PFS was 1.21 (95% CI, 0.93-1.57, P=.920) based on 224 events, and the hazard ratio for OS was 1.31 (95% CI, 0.93 -1.85; P=.937) based on 131 deaths.21
Nabil F. Saba, MD, director of the Head and Neck Medical Oncology Program at Winship Cancer Institute of Emory University in Atlanta, Georgia, pointed out that high doses of radiotherapy (70 Gy) with chemotherapy are highly toxic and may severely damage the activity of Teff cells and impair immune response modulation.
“Results of the JAVELIN trial,” he added, “must prompt us to evaluate the appropriate intensity of chemoradiotherapy that patients receive and the possibility that lower doses or less frequent schedules of radiotherapy may preserve immunomodulatory Teff-cell function.”
UCPVax
Telomerase reverse transcriptase is over-expressed in most cancer cells and plays a major role in cellular proliferation. UCPVax is a therapeutic vaccine containing telomerase-derived cancer peptides and induces a CD4-positive T-helper immune response against telomerase-expressing cells. The phase 2 VolATIL trial (NCT03946358) is evaluating the efficacy of UCPVax in combination with atezolizumab (Tecentriq) in previously treated patients who are HPV positive.
PD-1/PD-L1 Inhibitors in Previously Untreated Patients
An ongoing phase 2 trial (NCT04369937)is testing pembrolizumab plus HPV-16 vaccination and cisplatin in newly diagnosed, locally advanced, intermediate-risk HPV-16–associated HNSCC. Investigators are exploring sequential administration of the ISA101b vaccine before pembrolizumab and chemotherapy treatment, said Ferris. Phase 2/3 trials are evaluating immunotherapies in the maintenance setting, including pembrolizumab in KEYNOTE-412 (NCT03040999) and nivolumab in EA3161 (NCT03811015), along with chemotherapy or radiation in intermediate-risk HPV-16–positive HNSCC.22
Pembrolizumab is also being tested in AHEAD-MERIT (NCT04534205). This phase 2 trial is examining the agent for the first-line treatment of recurrent and/or metastatic HNSCC in combination with an investigational mRNA vaccine, BNT 113, a novel immunotherapy agent that targets the HPV-16 E6/E7 viral proteins.
Another trial is investigating CUE 101, a novel fusion protein designed to activate and expand tumor-specific T cells to treat HPV-driven malignancies. The phase 1 trial (NCT03978689) is accruing and will combine CUE 101 with pembrolizumab in patients with HPV-16–positive recurrent/metastatic HNSCC, including those who previously did not benefit from PD-1/PD-L1 inhibitors.
Immunotherapy with PD-1/PD-L1 inhibitors has advanced the treatment of advanced HNSCC, yet approved treatments do not distinguish between HPV-positive and HPV-negative OPCs. Improved under-standing of HPV-associated tumors shows that immune checkpoint inhibitors have superior clinical benefit in HPV-positive cancers. HPV-targeted vaccines are another promising strategy. Numerous studies combining both immunotherapy approaches in advanced HNSCCs are advancing to late-phase trials and showing higher response rates than those seen with either monotherapy. Results promise to radically change the way clinicians treat HPV-positive cancers through safer, less toxic, and more effective immunomodulatory approaches.
REFERENCES:
1. von Witzleben A, Wang C, Laban S, Savelyeva N, Ottensmeier CH. HNSCC: tumour antigens and their targeting by immunotherapy. Cells. 2020;9(9):2103. doi:10.3390/cells9092103
2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. doi:10.1038/s41572-020-00224-3
3. Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184. doi:10.1186/s40425-019-0662-5
4. Aggarwal C, Cohen RB, Morrow MP, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res. 2019;25(1):110-124. doi:10.1158/1078-0432.CCR-18-1763
5. Ferris RL, Blumenschein Jr G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi:10.1056/NEJMoa1602252
6. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi:10.1016/S1470-2045(16)30066-3. doi:10.1016/S1470-2045(16)30066-3
7. Massarelli E, Ferrarotto R, Glisson BS. New strategies in human papillomavirus-related oropharynx cancer: effecting advances in treatment for a growing epidemic. Clin Cancer Res. 2015;21(17):3821-3828. doi:10.1158/1078-0432.CCR-14-1329
8. Supplement Accelerated Approval. FDA. June 12, 2020. Accessed February 28, 2021.https://bit.ly/3kOnrvm
9. Gardasil 9. FDA. Updated August 21, 2020. Accessed February 28, 2021.https://bit.ly/3bQ7now
10. Paolini F, Curzio G, Cordeiro MN, et al. HPV 16 E5 oncoprotein is expressed in early stage carcinogenesis and can be a target of immunotherapy. Hum Vaccin Immunother. 2017;13(2):291-297. doi:10.1080/21645515.2017.1264777
11. Wang C, Dickie J, Sutavani RV, Pointer C, Thomas GJ, Savelyeva N. Targeting head and neck cancer by vaccination. Front Immunol. 2018;9:830. doi:10.3389/fimmu.2018.00830
12. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078-2088. doi:10.1016/S0140-6736(15)00239-1
13. Le Tourneau C, Cassier P, Rolland F, et al. TG4001 (Tipapkinogene sovacivec) and avelumab for recurrent/metastatic (R/M) human papilloma virus (HPV)-16+ cancers: clinical efficacy and immunogenicity. J Immunother Cancer. 2020;8(suppl 3):A841. doi:10.1136/jitc-2020-SITC2020.0793
14. Le Tourneau C, Cassier P, Rolland F, Salas S, Limacher J. TG4001 therapeutic vaccination combined with PD-L1 blocker avelumab remodels the tumor microenvironment (TME) and drives antitumor responses in human papillomavirus (HPV)+ malignancies. Ann Oncol. 2020;31(suppl 7):S1441-S1451. doi:10.1016/annonc/annonc392
15. Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838-1847. doi:10.1056/NEJMoa0810097
16. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542-1549. doi:10.1200/JCO.2016.70.1524
17. Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67-73. doi:10.1001/jamaoncol.2018.4051
18. Burtness B, Harrington KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3. Lancet. 2019;394(10212):1915-1928. doi:10.1016/S0140-6736(19)32591-7
19. Diehl MC, Lee JC, Daniels SE, et al. Tolerability of intramuscular and intradermal delivery by CELLECTRA adaptive constant current electroporation device in healthy volunteers. Hum Vaccin Immunother. 2013;9(10):2246-2252. doi:10.4161/hv.24702
20. Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi:10.1200/JCO.2016.68.1478
21. Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4(155):155ra138. doi:10.1126/scitranslmed.3004414
22. Machiels JP, Tao Y, Burtness B, et al. Pembrolizumab given concomitantly with chemoradiation and as maintenance therapy for locally advanced head and neck squamous cell carcinoma: KEYNOTE-412. Future Oncol. 2020;16(18):1235-1243. doi:10.2217/fon-2020-0184

