Oncoloytic viruses are an emerging class of cancer therapeutics that may provide a new strategy against malignant tumors.
 Oncoloytic viruses (OVs) are an emerging class of cancer therapeutics that may provide a new strategy against malignant tumors. These genetically modified viruses exert antitumor effects via various mechanisms of action, including selective replication in tumor cells, induction of immunogenic cell death, and stimulation of host antitumor immunity.1
Oncoloytic viruses (OVs) are an emerging class of cancer therapeutics that may provide a new strategy against malignant tumors. These genetically modified viruses exert antitumor effects via various mechanisms of action, including selective replication in tumor cells, induction of immunogenic cell death, and stimulation of host antitumor immunity.1
In addition to targeting tumors, OVs are hypothesized to have broad immunostimulatory effects as a result of their ability to stimulate the host adaptive immune response.2
Various molecular techniques are used to improve the tumor selectivity, safety, and immunogenicity of these viruses. For instance, OVs are engineered to express genes that promote antitumor activity, boost immune response, enhance sensitivity to radiation, improve targeted delivery. In this manner, OVs can produce potent immunostimulatory molecules at the site of the tumor, rather than systemically.3
The most common genetic modification is the addition of a granulocyte-macrophage colony-stimulating factor (GM-CSF) transgene, designed to recruit and mature local dendritic cells (DCs) and stimulate host immune responses. Upon viral replication, GM-CSF production leads to the subsequent priming of T cells with tumor-associated antigens (TAAs) released by oncolysis.1,3
OVs can also be designed to contain genes encod-ing prodrug-transforming enzymes such as cytosine deaminase and herpes simplex virus (HSV)-1 thymidine kinase. The patient is given a harmless prodrug, and the enzyme encoded by this so-called suicide gene converts it into a cytotoxic form in the tumor cell. This process ensures the selective killing of tumor cells expressing thymidine kinase and avoids viral replication in normal cells.1,4
“The OV delivers a 3-pronged attack through selective attack at the tumor sites, potent antitumor effects, and broad immune stimulation,” Mitesh J. Borad, MD, a medical oncologist at Mayo Clinic in Phoenix, Arizona, said in an interview with TargetedTherapies in Oncology (TTO).
OVs have been typically delivered through intratumoral administration, a method limited by the inability to reach visceral, brain, and metastatic lesions.2 In contrast, intravenous delivery poses the challenge of neutralization by host antibodies and dilution by the immune system. Thus, optimal drug delivery to the tumor site is a barrier to OV therapy.2
“One of the major challenges will be sufficient drug delivery to the tumor site,” Jorge Nieva, MD, an associate professor of clinical medicine at Keck School of Medicine, University of Southern California in Los Angeles, said during an interview with TTO. “A high concentration of product is required for local delivery, and there is a lot of variability in this procedure. Hence the need to improve intravenous delivery of oncolytic virus. In addition, it is possible to increase efficacy through tumor-adjacent delivery, which might require the involvement of interventional radiology colleagues,” he added.
HSV-Based OVs
Delivery of OVs to tumor cells is limited by host innate and adaptive immune responses. To over-come the resulting efficacy barriers, cell carriers can be used to transport OVs to tumor cells. Optimal cell carriers must be susceptible to virus infection, have the ability to locate the tumor tissue without being recognized by the immune system, and release progeny virus to attack distant cancer cells.5
Recombinant OVs have been observed to reverse immune suppression in the tumor microenvironment (TME), improve tumor immunogenicity, increase infitration of inflammatory cells, and have an antitumor effect.6
The most common OVs used in clinical research include adenovirus, HSV-1, reovirus, poxvirus, Newcastle disease virus, and measles virus.1
Large DNA viruses encode numerous immunomodulatory proteins that can evade the host immune system to enable viral replication and pathogenesis. Peptide and small interfering RNAs will need to be injected in large quantities at higher concentrations, which poses a major limitation to OV therapy.7
HSV is especially suited for use as an OV as it has multiple mechanisms to evade antiviral immune reaction by the host, such as expressing genes that allow it to evade the immune system, incapacitating immunoglobulins via viral glycoproteins, and inhibiting the production of cytokines and chemokines by infected cells.
Oncolytic HSV is widely used in clinical research, and its effects can be enhanced by combining it with immunotherapy and chemotherapy.6
Talimogene laherparepvec (T-VEC) is an attenuated HSV type 1 (HSV-1) OV that encodes GM-CSF for the treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.1 In the phase 3 OPTiM trial (NCT00769704), intratumoral T-VEC (Imlygic) induced meaningful tumor regression for at least 6 months in 16% of 436 patients with metastatic melanoma compared with 2% of patients in the control arm who received subcutaneous GM-CSF injection (P < .0001). The agent gained FDA approval in 2015.8,9
Positive results of T-VEC have boosted enthusiasm in the OV research field. In clinical practice, however, the availability of more effective therapies and inconvenient administration have limited the uptake of T-VEC.
Poxvirus
Poxviruses are large, double-stranded DNA viruses that can manipulate the host immune system. Vaccinia and myxoma viruses are both poxviruses but elicit different immune responses in humans, a factor that affects their pathogenic potential. Myxoma is a leporipoxvirus that is nonpathogenic in humans. Vaccinia is an orthopoxvirus that was used to vaccinate against smallpox and is potentially pathogenic in humans.10
Pexa-Vec is an attenuated DNA vaccinia virus with deletion of the thymidine kinase gene that is engineered to express GM-CSF. The PHOCUS phase 3 trial (NCT02562755) that evaluated the combination of Pexa-Vec with sorafenib was negative, and patient enrollment was halted at the interim futility analysis.1,11
The myxoma virus is a member of the Poxviridae family of DNA viruses with a large, double-stranded genome that permits engineering of multiple genes, is not pathogenic to humans and can be delivered intravenously. Investigators generated myxoma virus armed with transgenes that was designed to target different points in the cancer immunity cycle and to complement approved immune checkpoint inhibitors (ICIs).12
RNA helicase A/DHX9 is required for diverse RNA-related essential cellular functions and antiviral responses. Investigators have shown that during the late phase of myxoma virus replication in myxomainfected human cancer cells, DHX9 forms unique cytoplasmic structures termed antiviral granules that reduce protein synthesis in the cells. These granules are not formed, however, if myxoma DNA replication or late protein synthesis is blocked. This novel antiviral function of DHX9 that is recruited from the nucleus to the cytoplasm can be used to enhance OV therapy against cancer cells that would normally restrict myxoma.7
Adenovirus
Adenoviruses are excellent vectors for OV therapy. They are prone to aggressive replication within tumor cells and can be engineered for enhanced tumor selectivity.3 Similar to all OV counterparts, adenoviruses depend on the expression of specific receptors on the tumor surface for tumor entry, or tropism. Serotype 5 adenoviruses can be modified to contain arginylglycylaspartic acid (RGD) peptides in their fiber knobs to increase tumor tropism and reduce the toxicity of adenoviruses.3 DNX-2401, an RGD-modified adenovirus, was shown to induce durable survival (>3 years) in 20% of patients with glioma.13 Localized intratumoral administration of Delta-24-RGDOX, an oncolytic adenovirus that expresses the immune-stimulatory OX40 ligand, inhibited tumor growth in mice with disseminated subcutaneous and intracranial melanomas and boosted immune responses in virus-injected as well as untreated tumors.14 Investigators have recently engineered adenovirus to target αvβ6 integrin, which is absent from normal epithelium but overexpressed in head and neck squamous cell carcinoma (HNSCC) and ovarian, lung, esophageal, and cervical cancers. Because it stimulates angiogenesis and immune suppression, αvβ6 integrin represents a promising target for OVs.15
Phase 3 Trial of Adenovirus OV
Resistant non-muscle invasive bladder cancer is a challenging condition. The only surgical cure is cystectomy, a complex, life-changing procedure that many patients decline or are ineligible for due to comorbidities.17,18 Nadofaragene firadenovec (rAd-IFNa/Syn3) is an intravesical adenovirus vector-based gene therapy containing a human interferon alfa-2b transgene that showed promising efficacy in a phase 3 trial (NCT02773849) in patients with BCG-unresponsive nonmuscle-invasive bladder cancer (NMIBC). Complete response (CR) was noted in 53% of patients (n = 55/103) within 3 months, and the response was maintained in 46% of patients (n = 25/55) at 12 months. The adenovirus vector was administered by catheter in a repeat dosing fashion every 3 months at 3, 6, and 9 months. The study is ongoing and will include a monitoring phase.16
The most common grade 3 to 4 treatment-related adverse event (AE) was urinary urgency, and there were no treatment-related deaths. Intravesical rAd-IFNa/Syn3 was efficacious and well tolerated.
Results of this study were very encouraging and offer a feasible treatment that preserves the bladder and can fi t within the routine of a urology practice. This novel option may represent a new gold standard for treating this challenging disease.16,17,18, 19
Vesicular Stomatitis
Virus Some of the newest OVs are based on vesicular stomatitis virus (VSV), including VSV-IFNβ-NIS, a therapeutic developed by investigators at Mayo Clinic, said Stephen J. Russell, MD, PhD, a professor of medicine at Mayo Clinic, Rochester, Minnesota.
“VSV-IFNβ-NIS induced good responses in patients with T-cell lymphoma, including a CR that was sustained for more than 1 year,” Russell said. “There were encouraging results from preliminary studies that support the use of a single dose of OV.” This avoids the need for repetitive dosing and preventing neutralization by the immune system. Ongoing phase 2 trials are evaluating single intravenous doses of the VSV-based OV Voyager-V1 in combination with immunotherapy.
Single-dose intravenous Voyager-V1 is being investigated in combination with cemiplimab (Libtayo) in different cancer indications (NSCLC, hepatocellular carcinoma, endometrial cancer, and melanoma; NCT04291105) and in combination with pembrolizumab in NSCLC and HNSCC (NCT03647163).20
OVs in Combination With Immunotherapy
Studies of OVs in combination with immunotherapy have shown an increase in response rates, providing rationale for further trials using this strategy. OVs have the potential to boost IC inhibition. Tumors with low amounts of immunological cells can be immune activated by oncolytic adenoviruses, causing tumors to become “hot” and boosting the effects of ICIs.3 Investigators have engineered OVs to express PD-L1 inhibitors and therefore block PD-L1 expressed on tumor cells and immune cells, this enhances the activity of neoantigen-specific T-cell responses and potentiates the antitumor effect. This is particularly beneficial in patients who are insensitive to PD-1/PD-L1 blockade.5
Positive phase 1 results were reported from a study (NCT02263508) evaluating T-VEC and pembrolizumab (Keytruda) in advanced melanoma, resulting in a 62% confirmed objective response rate (ORR), a 33% CR rate, and increased CD8-positive T cells and PD-L1 protein expression in responders.3,21 In a phase 1/2 trial in patients with advanced melanoma (NCT01740297), T-VEC in combination with ipilimumab (Yervoy) produced a higher ORR compared with ipilimumab alone (39% vs 18%, respectively; P = .002) with no increase in AEs.3,22
“Research in oncolytic viruses has been ongoing for the last 15 to 20 years, and the possibility to combine these agents with ICIs will clearly improve the response against TAAs along with improved T-cell tumor response. Before checkpoint blockage, this would not have been possible,” said Nieva.
On the combination of OV and immunotherapy, Russell said, “OV is the perfect partner for ICIs, as the former acts like a tumor vaccine that stimulates T cells and boosts response to TAAs and leaves an immune trail for continued action of the immune system.” Russell added that investigators hoped to combine a single intravenous dose with immune checkpoint inhibition preclinically and get cure responses compared with either approach alone.
Adenoviral therapy in combination with immunotherapy has shown positive results in patients with glioblastoma in the phase 2 CAPTIVE/KEYNOTE-192 study (NCT02798406). DNX-2401, also referred to as tasadenoturev. Patients with recurrent glioblastoma received a combination of DNX-2401 and pembrolizumab. Among 42 patients, the median overall survival (OS) was 12.5 months, and 4 patients were still alive at 23 months. The 18-month OS rate was 20.2%. Results were presented in a late-breaking presentation at the Society for NeuroOncology 2020 Annual Meeting. A single 1.0-mL intratumoral dose of DNX-2401 was followed 7 to 9 days later by 200-mg intravenous pembrolizumab every 3 weeks . Treatment continued for 105 weeks or until disease progression or unacceptable toxicity as described in the CAPTIVE trial (NCT02798406).23
OVs in Combination With Adoptive Cell Therapy
OVs have the potential to transform the immunosuppressive TME into an immunostimulatory environment that permits T-cell entry. OV-induced tumor necrosis may also lead to the release of TAAs, promoting the priming and activation of neoantigen-specific T cells. Findings from preclinical studies have shown that OVs armed with ICIs, cytokines, or bispecific T-cell engagers enhanced the antitumor functions of CAR T cells.4
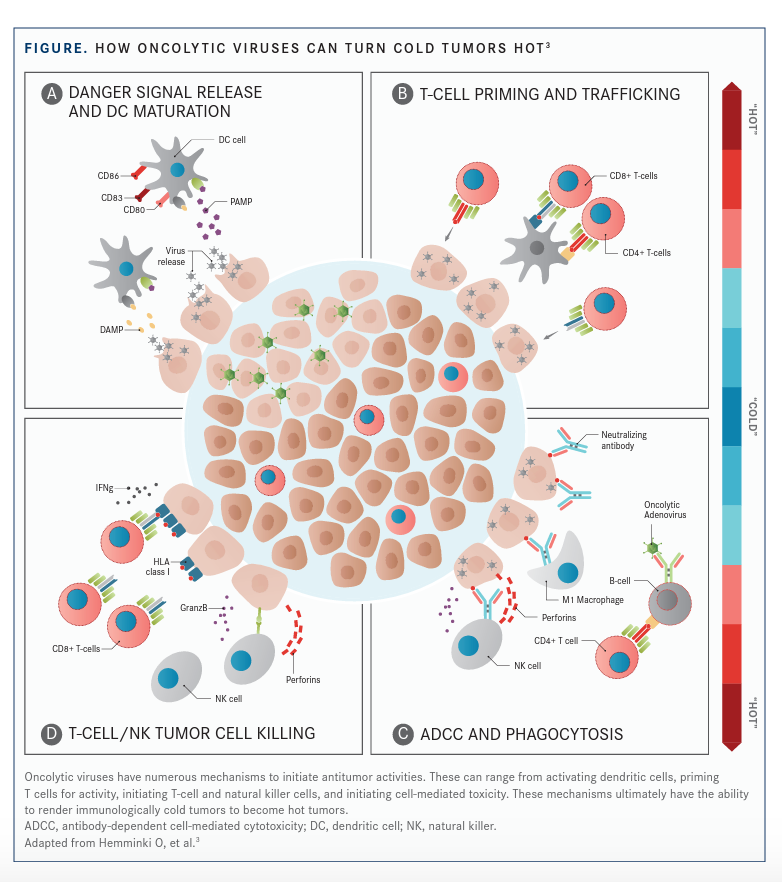
OVs can activate Toll-like receptors and innate immune response pathways to initiate proinflammatory cascades that alter the balance of inhibitory and activating immune cells. OVs have been shown to promote T-cell infiltration and PD-L1 upregulation and to render immunologically cold tumors hot (FIGURE3).24,25
Loading CAR T cells with OV pelareorep, a wild-type reovirus, improved their efficacy in solid tumor mouse models in a preclinical study presented at the CAR-TCR Summit Europe 2021. Pelareorep made tumors susceptible to ICIs and CAR T cells, and it overcame limitations of CAR T in solid tumors that result from insufficient infiltration in immunosuppressive environments. Pelareorep can reverse an immunosuppressive TME, and combining it with CAR T may enable success in solid tumors.26 In a proof-of-concept study, investigators explored the potential of a vaccinia OV expressing a CD19t (truncated variant of CD19) transgene to target CD19-specific CAR T cells to solid tumors. The successful expression of CD19t by infected tumor cells was demonstrated, as were potent antitumor responses in human tumor xenograft and mouse tumor models.27
“In oncolytic viral therapy 2.0, innovative techniques will allow us to overcome limitations of OV delivery, preexisting immunity, and reduced efficacy in monotherapy,” said Borad.
OVs represent a new class of cancer therapy with the potential to induce tumor cell death through the channeling of both innate and tumor-specific adaptive immune responses. OVs can be modified to improve antitumor activity by enhancing antitumor immunity through expression of transgenes that increase direct cell killing or enhance host immunity.2,5
A limited number of OVs are approved for clinical treatment. OV therapy does not have a stable curative effect, and its effect is inconsistent among individuals. Combining OVs with immunotherapy, using cell carrier construction or genetic engineering, can improve outcomes in patients.5
“In the post–COVID-19 era, it will be important to reassure community oncologists about the lack of disease transmission from these viruses. OVs are modified to be attenuated by deletion of thymidine kinase and hence cannot replicate in normal cells but only in tumor cells that express thymidine kinase,” Nieva said. “Ultimately, each of these therapies will be evaluated individually for a specific clinical use and indication. OVs are highly specific therapies targeted to specific TAAs. The combination of OVs with CAR T cells holds great promise,” he added.
REFERENCES:
1. Macedo N, Miller DM, Haq R, Kaufman HL. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer. 2020;8(2):e001486. doi:10.1136/jitc-2020-001486
2. Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6(1):140. doi:10.1186/s40425-0180458-z
3. Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13(1):84. doi:10.1186/s13045-020-00922-1.
4. Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics. 2019;15:234-247. doi:10.1016/j.omto.2019.10.007
5. Cao GD, He XB, Sun Q, et al. The oncolytic virus in cancer diagnosis and treatment. Front Oncol. 2020;10:1786. doi:10.3389/ fonc.2020.01786.
6. Ma W, He H, Wang H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018;19(1):40. doi:10.1186/s12865018-0281-9
7. Rahman MM, Gutierrez-Jensen AD, Glenn HL, Abrantes M, Moussatche N, McFadden G. RNA helicase A/DHX9 forms unique cytoplasmic anti-viral granules that restrict oncolytic myxoma virus replication in human cancer cells. J Virol. Published online May 5, 2021. doi:10.1128/JVI.00151-21
8. Andtbacka RHI, Collichio FA, Amatruda T, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-SCF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31 (suppl 18): LBA9008. doi: 10.1200/jco.2013.31.18_suppl.lba9008
9. Sampath P, Thorne SH. Arming viruses in multi-mechanistic oncolytic viral therapy: current research and future developments, with emphasis on poxviruses. Oncolytic Virother. 2013;3:1-9. doi: 10.2147/OV.S36703
10. Cao H, Dai P, Wang W, et al. Innate immune response of human plasmacytoid dendritic cells to poxvirus infection is subverted by vaccinia E3 via its Z-DNA/RNA binding domain. PLoS One. 2012;7(5):e36823. doi:10.1371/journal.pone.0036823
11. GEN: Genetic Engineering & Biotechnology News. Pexa-vec/ Nexavar combination fails phase III Trial in liver cancer. News release. Accessed June 22, 2021. https://bit.ly/3vS5ShR.
12. Torres-Domingues LE, Franco LS, Abrantes et al. Armed myxoma virus demonstrates therapeutic effi cacy in xenograft models. J Immunother Cancer. 2020;8. doi:10.1136/jitc-2020SITC2020.0596.
13. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic eff ects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419-1427. doi:10.1200/ JCO.2017.75.8219
14. Jiang H, Shin DH, Nguyen TT, et al. Localized treatment with oncolytic adenovirus delta-24-RGDOX induces systemic immunity against disseminated subcutaneous and intracranial melanomas. Clin Cancer Res. 2019;25(22):6801-6814. doi:10.1158/10780432.CCR-19-0405
15. Uusi-Kerttula H, Davies JA, Thompson JM, et al. Ad5NULL-A20: A tropism-modifi ed, αvβ6 integrin-selective oncolytic adenovirus for epithelial ovarian cancer therapies. Clin Cancer Res. 2018;24(17):4215-4224. doi:10.1158/1078-0432.CCR18-1089
16. Boorjian SA, Alemozaff ar M, Konety BR, et al. Intravesical nadofaragene fi radenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22(1):107-117. doi:10.1016/S1470-2045(20)30540-4
17. Cook J, Peng KW, Geyer SM, et al. Clinical activity of systemic VSV-IFNβ-NIS oncolytic virotherapy in patients with relapsed refractory T-cell lymphoma. J Clin Oncol.2021;39:(suppl. 15):2500. doi:10.1200/JCO.2021.39.15_suppl.2500
18. Kulkarni GS. Nadofaragene fi radenovec: a new gold standard for BCG-unresponsive bladder cancer? Lancet Oncol. 2021;22(1):8-9. doi:10.1016/S1470-2045(20)30586-6
19. Broderick J. Nadofaragene fi radenovec published data show strong effi cacy in BCG-unresponsive NMIBC. Urology Times. Accessed June 22, 2021. https://bit.ly/2UxmfU0.
20. Clinical program: a new modality for the treatment of cancer. Vyriad. Accessed June 7, 2021. https://bit.ly/3xcm8eG.
21. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infi ltration and improves anti-PD-1 immunotherapy. Cell. 2018;174(4):1031-1032. doi:10.1016/j. cell.2018.07.035.
22. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the effi cacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658-1667. doi:10.1200/ JCO.2017.73.7379
23. DNAtrix announces positive data from phase 2 CAPTIVE/ KEYNOTE-192 study with DNX-2401 in patients with recurrent glioblastoma highlighted in an oral late-breaking presentation during Society for Neuro-oncology (SNO) annual meeting. News release. Accessed June 10, 2021. https://prn.to/3cwvlGP
24. Evgin L, Huff AL, Wongthida P, et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat Commun. 2020;11(1):3187. doi: 10.1038/s41467-020-17011-z
25. Oncolytics Biotech reports preclinical data demonstrating the synergistic anti-cancer activity of pelareorep combined with CAR T cell therapy in sold tumors. Accessed June 23, 2021. https://prn.to/3xQv2ih
26. Park AK, Fong Y, Kim SI, et al. Eff ective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med. 2020;12(559):eaaz1863. doi:10.1126/ scitranslmed.aaz1863
27. McGrath K, Dotti G. Combining oncolytic viruses with chimeric antigen receptor T cell therapy. Hum Gene Ther. 2021;32(34):150-157. doi:10.1089/hum.2020.278

